Your cart is currently empty!
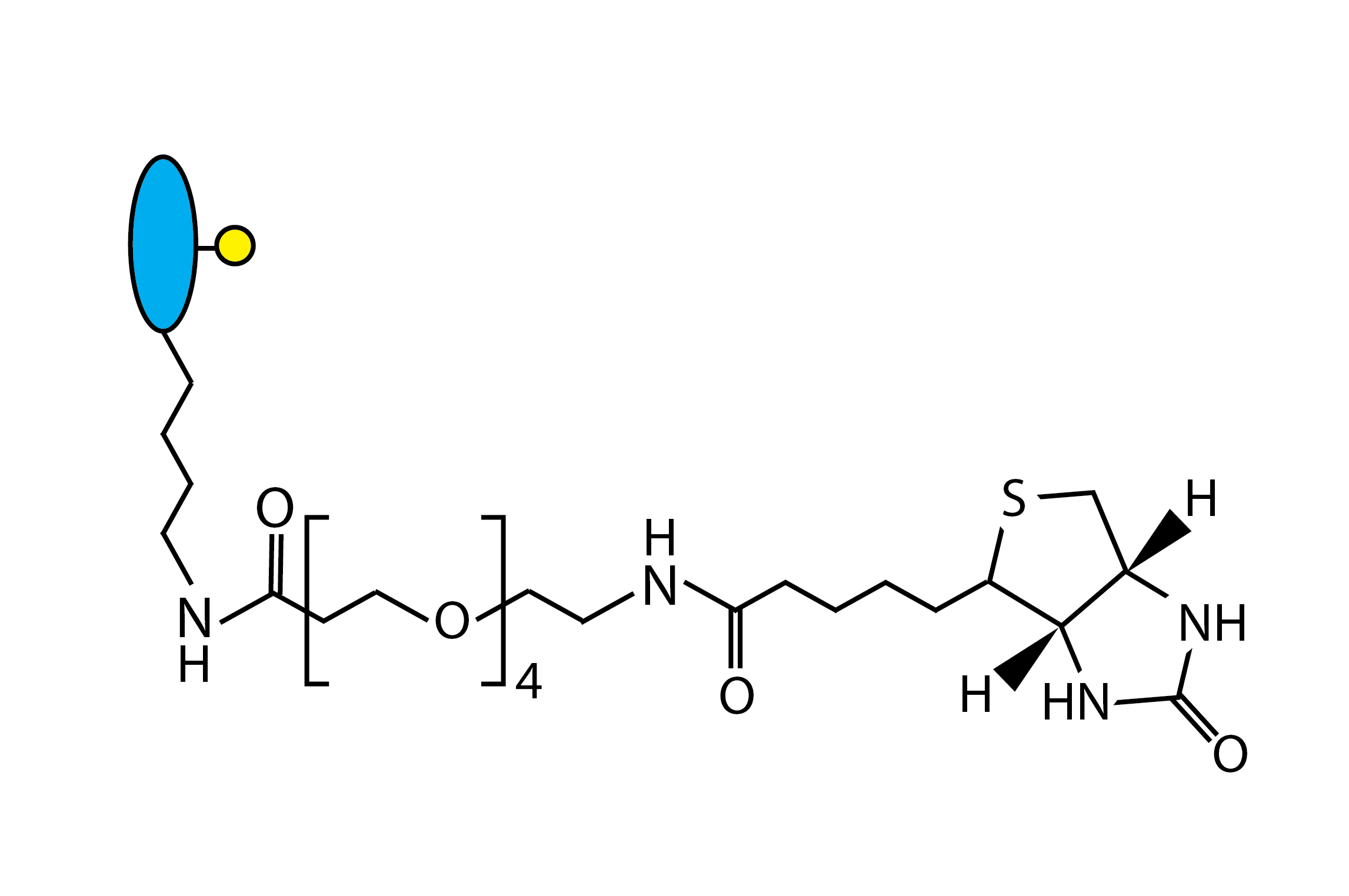
oYo-Link® Single-Biotin
Site-specific labeling of compatible antibodies with up to two labels of Biotin for antibody surface immobilization on streptavidin-coated surfaces.
Site-specific labeling of compatible antibodies with up to two labels of Biotin for antibody surface immobilization on streptavidin-coated surfaces.
oYo-Link® Antibody Biotin Labeling Reagents
For research use only.
oYo-Link® Single-Biotin enables site-specific labeling of compatible antibodies with up to two labels of Biotin for antibody surface immobilization on Streptavidin-coated surfaces. Site-specific immobilization ensures proper antibody orientation, which can improve sensitivity, stability and longevity of capture antibodies for ELISA. Simply mix the oYo-Link Single-Biotin with desired antibody followed by photocrosslinking for conjugation in 30-sec hands on time, 2 hours total.
Requirements: Photo-Crosslinking Device
Quote or Bulk Orders? Click here.
oYo-Link® Single-Biotin is recommended for the immobilization of antibodies on Streptavidin-coated surfaces (or coated w/ other biotin-binding proteins). oYo-Link® Single-Biotin enables the site-specific labeling of oYo-Link®-compatible antibodies with a biotin to the IgG heavy chain. The use of a single biotin maximizes mobility and antigen binding density of surface-immobilized antibodies.
Site-specific immobilization can lead to an improvement in sensitivity, stability and longevity of capture antibodies. While the magnitude of improvement is dependent on various parameters, such as antibody binding properties, uniform antibody orientation on a surface can result in an increase in sensitivity in enzyme-linked immunosorbent assays (ELISA) by enabling the capture of more antigens per unit area.
Generate antibody-biotin conjugates in only 30-seconds hands on time, 2 hours total.
The antibody labeling procedure simply requires mixing oYo-Link® Single-Biotin with the desired antibody followed by photocrosslinking with non-damaging black light (365 nm).
Photocrosslinking can be carried out in most buffers, including those containing amine-containing molecules (e.g. TRIS, glycine) or storage proteins (see Buffer Compatibility Table).
Applications: Conjugation of capture antibodies for Streptavidin-Coated Surface Immobilization in Direct ELISA; Also for Sandwich-ELISA
Requirements: To label your compatible antibodies with oYo-Link®, you will need a light source emitting at 365nm (6-10W). Purchase AlphaThera’s LED Photo-Crosslinking Device here, or use a compatible device.
Placing an order: For credit card purchases, proceed with checkout on website. To request a quote click here.
Technical Support: For any technical questions please contact our technical support team here.
Webinar: Simple, fast, site-specific antibody labeling with oYo Link®
Watch our short introduction to AlphaThera’s oYo-Link® site-specific antibody conjugation technology and learn how to streamline your assays with these superior labeling products.
Antibody Conjugation User Manual
Read our antibody conjugation usual manual for instructions on how to easily conjugate your antibody with oYo-Link products.
Antibody Surface Immobilization with oYo-Link Single-Biotin
Guides users on how to immobilize their antibodies in 96-well plates for subsequent antigen capture assays.
Demo: oYo-Link® Antibody Conjugation
Find out how to site-specifically label your antibody with just two steps: mix and illuminate. 30 seconds hands-on time, 2 hours total.
oYo-Link® Product Brochure
Download our product brochure
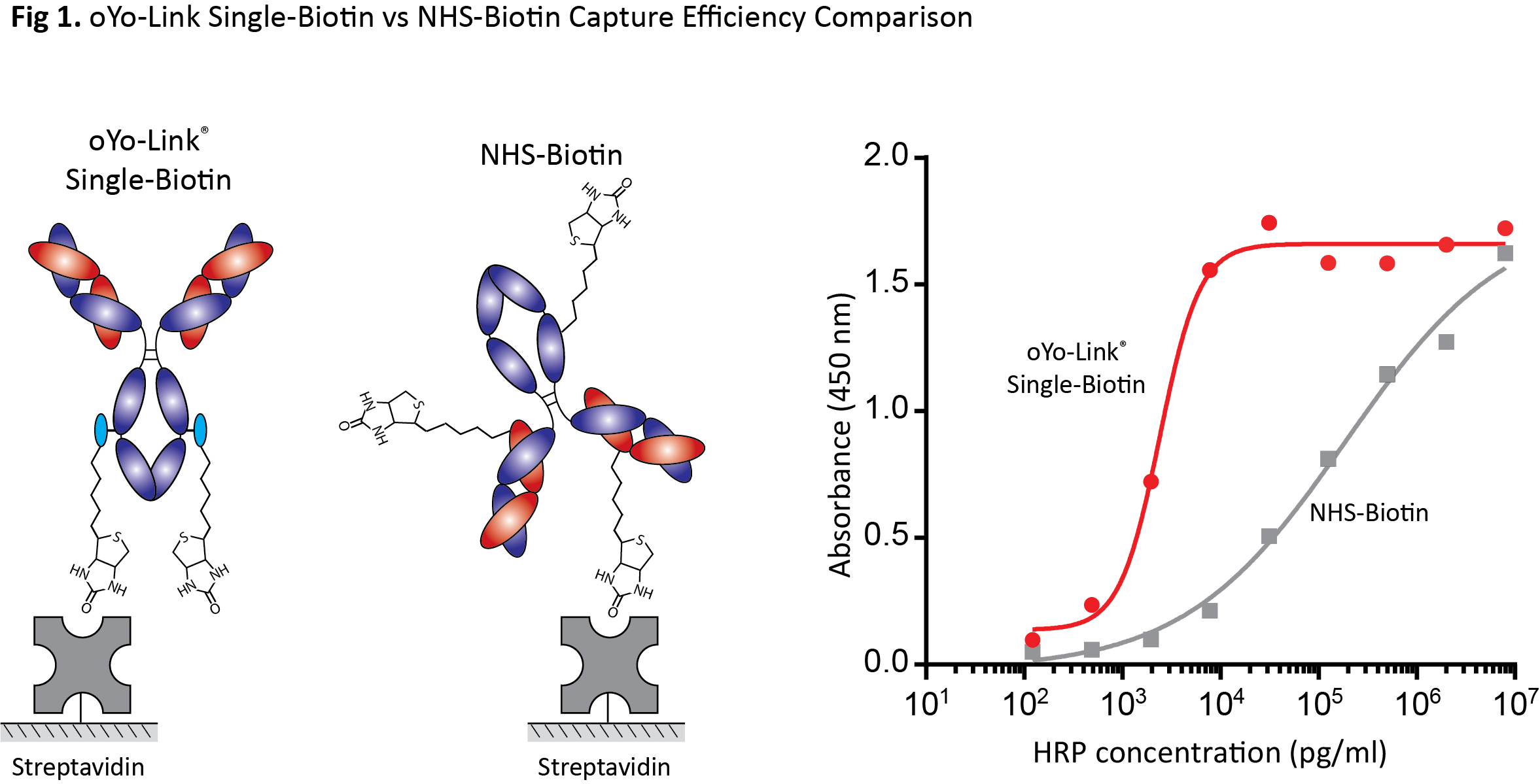
Fig 1. oYo-Link Single-Biotin vs NHS-Biotin Capture Efficiency Comparison
oYo-Link Single-Biotin vs NHS-Biotin Capture Efficiency Comparison: Anti-Horseradish Peroxidase (HRP) antibody was labeled with either oYo-Link Single-Biotin or NHS-Biotin and immobilized on a streptavidin-coated microplate. Site-specific immobilization of the anti-HRP antibody labeled with oYo-Link Single-Biotin led to a significant improvement in the capture of HRP, owing to the proper orientation of the antibodies on the surface. In contrast, when antibodies were randomly labeled with NHS-Biotin, the capture efficiency was comparatively worse, due to the improper orientation of some antibodies on the surface and the corresponding reduction in the number of available HRP binding sites.
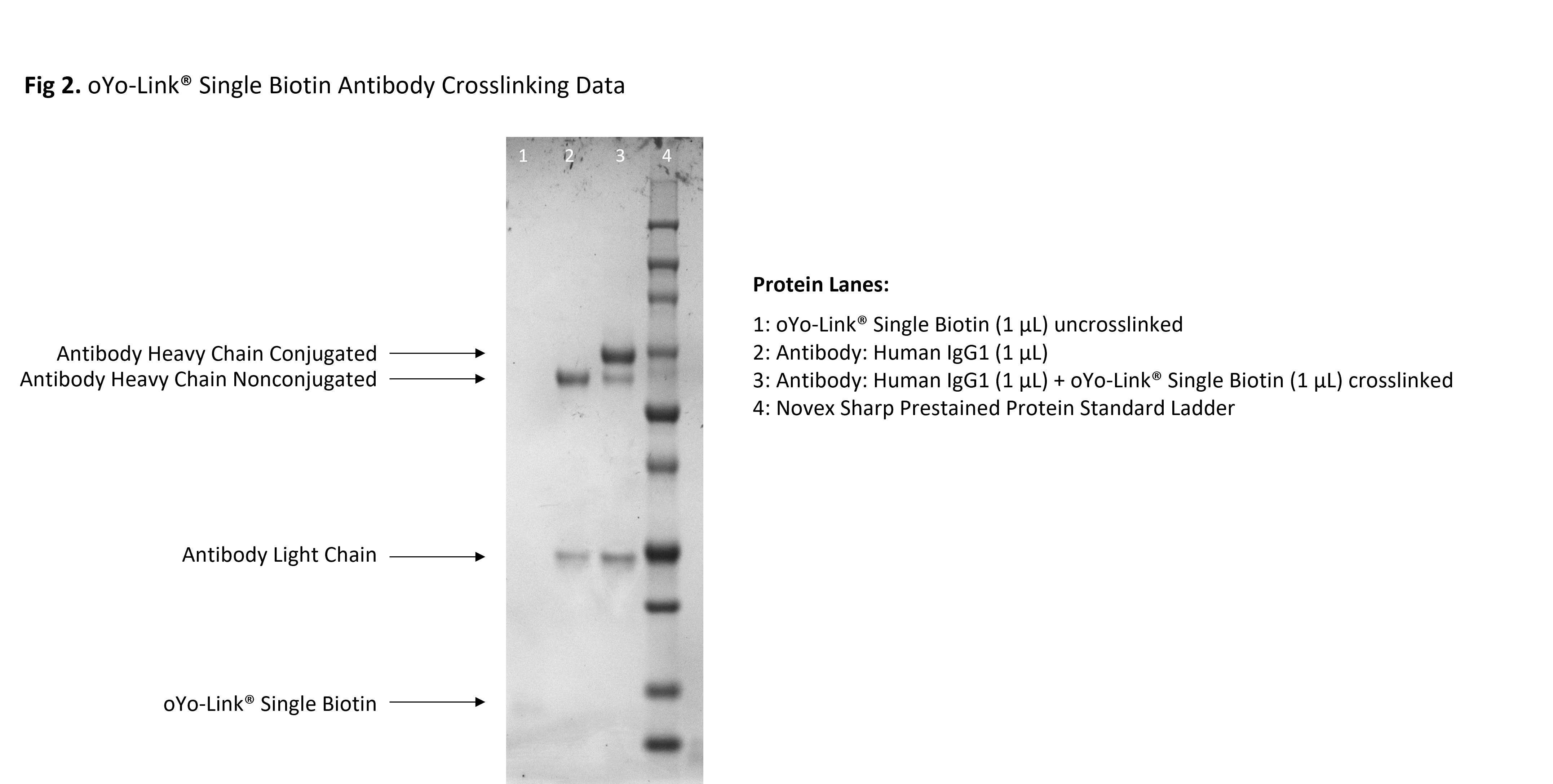
Fig 2. oYo-Link® Single Biotin Crosslinking Data
oYo-Link Single-Biotin Photo-crosslinking Efficiency shown by SDS-PAGE: using a 4-12% Tris Gel shows an upwards shift of the antibody heavy chain following site-specific photo-crosslinking with oYo-Link. *Note the size and migration for each unreacted oYo-Link product will differ.
Specifications:
| Product Name | oYo-Link® Single Biotin |
| Catalog # | AT4001 |
| Lot # | See the Spec Sheet shipped with product. |
| MSDS | |
| Antibody and Buffer Compatibility | View the oYo-Link Antibody & Buffer Compatibility Table here. Note: To label mouse IgG1 antibody, you must order the oYo-Link mIgG1 products, which will only label mIgG1 antibody. |
| Molecular Weight | 8000 g/mol |
| Concentration | 270 µg/mL |
| Molar Concentration | 33 µM |
| Storage Buffer | 1 x PBS |
| Shipping Condition | This product is shipped as a dried clear or white pellet at ambient temperature. |
| Reconstitution | Before opening, briefly centrifuge each tube to ensure that the entire pellet is at the bottom. For all product tubes: Add 100 µL sterile ddH2O and vortex tube briefly until fully dissolved. |
| Storage and Stability | The dried product can be stored at 4°C or -20°C upon receipt. Following reconstitution, the product can be stored at 4°C for short-term durations (< 1 week) and -20°C to -80°C for longer time-spans. |
| Conjugation Instructions | After reconstitution, each product tube is capable of labeling up to 100 µg of antibody. Please find and follow the Antibody Conjugation User Manual here |
| Other NotesoYo-Link® Single Biotin | Disposal of product should be performed in compliance with all applicable federal, state, and local regulations. This product does not meet hazard classification criteria based on evaluations made by our company under the OSHA Hazard Communication Standard, 29 CFR 1910.1200. |
Will oYo-Link work with my antibody?
Please check the antibody compatibility table. Note: oYo-Link mIgG1 can be used to label mouse IgG1 antibody. Currently, oYo-Link mIgG1 is only available for certain products. Please check the product webpage. oYo-Link mIgG1 only labels mouse IgG1 and is not compatible with other antibodies.
Can oYo-Link be used to label antibodies in the presence of albumin and/or Tris?
Yes, since oYo-Link specifically bind to the heavy chain of IgG, it works in the presence of both albumin and Tris. oYo-Link is compatible with all common buffers. See the full buffer compatibility table here.
If an oYo-Link product has accidentally been left at room temperature for a week, will it still work?
With the exception of oYo-Link DBCO, all oYo-Link products are shipped as white or clear pellets and are very stable. While we recommend cold storage, they will not exhibit any loss of activity if left at room temperature for a week. oYo-Link DBCO must be shipped and stored cold (-20°C) immediately upon arrival, otherwise there will be a loss in activity.
Does AlphaThera offer conjugation service?
Yes. If you are interested in our conjugation service, please contact us at support@alphathera.com. For custom conjugation services, you will need to send us your antibody. The conjugation and purification fee depends on the size and specifications of each order. Purification is not required for most applications.
How many oYo-Link labels will be conjugated to each antibody?
For most subclasses and species of antibody, oYo-Link will result in the conjugation of 2 labels per antibody (maximum), one label per heavy chain. For example, ~95% of human IgG and Rabbit IgG will have two labels per antibody. However, there are a few antibodies such as mouse IgG2b and goat IgG, where the conjugation is slightly less efficient. In this case, 60 to 80% of the antibody will be labeled and have a mixture of 0, 1 and 2 labels per antibody. See the antibody conjugation efficiency here
How do I know if oYo-Link was successfully conjugated to my antibody?
Antibody conjugation can be checked on SDS-PAGE gel. An example is present in the supporting data on each product page.
Does 365nm UV illumination damage the antibody and its binding affinity?
The conjugated and unconjugated antibody binding affinities have been tested via both ELISA and cell binding assays and don’t show any difference.
Do I need to remove free oYo-Link after conjugating to antibody?
For most immunoassay applications, purification is not necessary. In our standard recommended protocol, the molar ratio of oYo-Link-to-antibody is 5:1. These conditions ensure that the antibody conjugation efficiency reaches its maximum; however there will be a slight excess remaining in your antibody mixture. This is usually removed during immunoassay washing steps. However, if your application requires high antibody-conjugate purity, please contact us for more information on this process and for a detailed protocol.
For oYo-Link click-chemistry products, should I perform the click reaction before conjugation?
When working with oYo-Link Azide (Catalog #: AT3002) and oYo-Link DBCO (Catalog #: AT3003), it is required to perform the click chemistry coupling reactions with the molecule of interest first, prior to photo-crosslinking to an antibody. This is to avoid UV illumination damage to the click moiety and to avoid diluting oYo-Link prior to the reaction to maximize the reaction efficiency. For oYo-Link Tetrazine (Catalog #: AT3004) and oYo-Link Thiol (Catalog #: AT3001); it is recommended to perform the coupling reaction before photo-crosslinking. This is to avoid diluting oYo-Link prior to the coupling reaction to maximize the reaction efficiency.
What is the lowest amount of antibody that can be labeled by oYo-Link reagents?
We recommend working with at least 1 µg of antibody; however, it is possible that even lower amounts of antibody can be labeled.
Can antibodies that are highly dilute be labeled by oYo-Link reagents?
Yes, antibodies can be efficiently labeled even if diluted to concentrations as low as 50 µg/mL.
oYo-Link® Biotin Advantage
- Site-Specific & Covalent Reaction. Covalent labeling of an antibody’s Fc region only, with no impact on antibody binding, ensures experimental reliability and reproducibility.
- Improved sensitivity, stability and longevity of capture antibodies.
- <30 seconds “hands-on” time, 2 hours total. Two simple steps, Mix and Illuminate.
- Label as little as 1 µg of antibody per reaction.
- Label diluted antibody solutions, as low as 50 µg/mL.
- Compatible with all common buffers, including BSA, Tris & Azide.
- >90% labeling efficiency of most antibodies.
- After resuspension, aliquot and save for future reactions.
Please Select Epitope Tag Options
4 EPITOPE TAGS: Please select only 4 Tags Below:
Each tag will be able to label the amount of Ab you selected on the previous page.